APOLIPOPROTEINS
General Information for Lipoproteins and Apolipoproteins
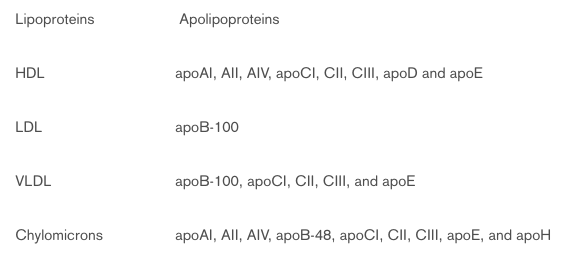
Plasma lipoproteins classes can be defined according to the densities at which they are isolated, as high (HDL), low (LDL), intermediate (IDL), very low-density lipoproteins (VLDL), and the chylomicrons. In general, lipoprotein particles range in size from 10 to 1000 nm. They are composed of a hydrophobic core containing cholesteryl esters, triglycerides, fatty acids and fat-soluble vitamins. The surrounding hydrophilic layer is composed of various apolipoproteins, phospholipids and cholesterol.
Apolipoproteins can be isolated by delipidation from the lipoprotein, and a number of preparative methods, such as gel filtration or DEAE chromatography have been established.
1. Apolipoprotein AI (ApoAI)
ApoAI comprises approximately 70% of the protein moiety in HDL. It is a single polypeptide chain consisting of 243 amino acid residues without disulfide bound and with glutamic acid as the C-terminal residue and aspartic acid as the N-terminal residue. The molecular weight is reported to be 28 kDa (Brewer et al., 1978). ApoAI activates lecithin-cholesterol (LCAT) acyltransferase, which is responsible for cholesterol esterification in plasma. ApoAI levels may be inversely related to the risk of coronary disease.
2. Apolipoprotein AII (ApoAII)
ApoAII comprises 25% of ApoAI in HDL. It exists in human plasma as a dimer of 2 identical chains of 77 amino acid residues, joined by disulfide. The molecular weight is reported to be 8.7 kDa for a single chain (Brewer et al., 1972). Studies on mouse reported that apoAII may be proatherogenic (Warden et al., 1993); however, case-control Study in the large European Prospective Investigation demonstrated that plasma apoAII concentrations were strongly inversely correlated with CHD events (Birjmohun et al., 2007).
3. Apolipoprotein B (ApoB)
ApoB exists in human plasma in two isoforms, ApoB-48 (Chen et al., 1987) and ApoB-100 (Wei et al., 1985, Yang et al., 1986a; 1989a,b; 1990; Chen et al., 1986; Yang et al., 1990, Yang and Pownall 1992). ApoB-100 is the major physiological ligand for the LDL receptor. ApoB100 is a large monomeric protein, containing 4536 amino acids (m.w. 515 kDa, Yang et al., 1986b). ApoB-100 is synthesized in the liver and is required for the assembly of VLDL. It is found in LDL and VLDL after the removal of the apoA, E and C. ApoB-48 is present in chylomicrons and their remnants. It is essential for the intestinal absorption of dietary lipids. ApoB levels correlate with the risk of coronary disease. ApoB-48 is synthesized in the small intestine. It comprises approximately half of the N-terminal region of ApoB-100 and is the result of posttranscriptional mRNA editing by a stop codon in the intestine not found in the liver.
4. Apolipoprotein CI (ApoCI)
ApoCI contains 57 amino acid residues and the m.w. is 6.6 kDa (Jackson et al., 1974). ApoCI has been found to activate LCAT (Liu and Subbaiah 1993) and to inhibit cholesterol ester transferase that can potentially regulate several lipase enzymes (Poensgen, 1990, Conde-Knape et al., 2002; Berbee et al., 2005.)
5. Apolipoprotein CII (ApoCII)
ApoCII contains 78 amino acid residues. The m.w. is 8.5 kDa (Jackson et al., 1977). ApoCII activates lipoprotein lipase that hydrolyzes fatty acids from triacylglycerols in chylomicrons.
6. Apolipoprotein CIII (ApoCIII)
ApoCIII contains 79 amino acid residues. The m.w. is 8.7 kDa (Brewer et al., 1974). It may inhibit the activation of lipoprotein lipase by apoCIII. ApoCIII is a constituent of both apoB- and apoAI-containing lipoproteins in the circulation. ApoCIII plays a pivotal role in regulating the plasma metabolism of VLDL, IDL, and LDL, primarily by inhibiting receptor-mediated uptake of these lipoproteins by the liver (Sehayek and Eisenberg 1991, Aalto-Setala et al., 1992, Zheng et al., 2007)
7. Apolipoprotein E (ApoE)
ApoE contains 299 amino acid residues. It is a 34-37 kDa glycosylated protein (Rall et al., 1983). ApoE is involved with triglyceride, phospholipid, cholesteryl ester, and cholesterol transport in and out of cells and is a ligand for LDL receptors. ApoE has also been implicated in immune and nerve degeneration. It has been found to suppress lymphocyte proliferation. Late-onset familial and sporadic Alzheimer disease patients have been found to have a higher occurrence of one of the three common ApoE isoforms, ApoE4. The ApoE4 isoform has been detected in senile plaques and neurofibrillary tangles of Alzheimer disease patients. ApoE4 is associated with rapid chylomicron-remnant clearance and increased total cholesterol levels.
8. Apolipoprotein (a) [apo (a)]
The plasma concentration of human lipoprotein[a], Lp[a], is highly correlated with coronary artery disease. The protein moiety of Lp[a], apoLp[a], consists of two apoproteins, apo[a] and apoB-100, linked by one or more disulfide bonds(s). Apo[a], the protein unique to Lp[a], exists in polymorphic forms that exhibit different apparent molecular weights ranging from 419 kDa to 838 kDa (Gaubatz et al., 1983; 1990, 1993).
9. Plasminogen
Plasminogen contains 810 amino acid residues. It is a single chain glycoprotein with m.w. 90 kDa (Robbins et al., 1967), soluble in water; prepared from plasma that has been shown by certified test to be negative for HBsAg and for antibodies to HIV and HCV. Plasminogen is the inactive precursor of the protease plasmin. Plasminogen is activated by the action of either tissue plasminogen activator (tPA), which primarily activates the fibrinolytic (thrombolytic) activity of plasmin, or urokinase plasminogen activator (uPA), which is associated with extracellular matrix remodeling and cell migration.
10. C-Reactive Protein (CRP)
Human C-Reactive Protein (CRP) is an important biomarker for predicting of future cardiovascular events, such as heart attack and stroke (Koenig et al., 1999, Jenny et al., 2007, Kabagambe et al. 2011). CRP is an acute phase protein produced by the liver. It is a member of the pentraxin family of proteins with five identical nonglycosylated subunits of 206 amino acids each (m.w. 23 kDa) (Agrawal et al., 2009). Among other markers of inflammation, CRP has shown the strongest association with cardiovascular events (Marsik et al., 2008, Kones et al., 2010). Clinical studies demonstrated that coronary mortality among patients with unstable angina and elevated CRP is significantly higher comparing with the patients without elevated CRP. It is an important biomarker for detecting individuals at high risk of plaque rapture. Our series of CRP products including Human C-Reactive Protein (CRP), Rabbit Anti-Human-CRP, Goat ant-Human-CRP, and its modified conjugated antibodies, such as HRP, Biotin, and FITC products can be used for detecting CRP levels to evaluate the risk of heart attack.
11. Irisin
The exercise-inducible myokine Irisin (Boström, et al. 2012) is secreted by cleavage from the transmembrane precursor, FNDC5. Circulating Irisin govern a wide variety of cellular processes relevant to browning, angiogenesis, wound healing, bone mass, and metabolism.
References:
Aalto-Setala , K., E. A. Fisher , X. Chen , T. Chajek-Shaul , T. Hayek , R. Zechner , A. Walsh , R. Ramakrishnan , H. N. Ginsberg , and J. L. Breslow . “ Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles.” J. Clin. Invest. 90 (1992): 1889 – 1900.
Agrawal, Alok, Prem Prakash Singh, Barbara Bottazzi, Cecilia Garlanda, and Alberto Mantovani. "Pattern Recognition by Pentraxins." Advances in Experimental Medicine and Biology 653 (2009): 98-116.
Berbee, J.F., C.C. van der Hoogt, D. Sundararaman, L.M. Havekes, and P.C. Rensen. “Severe hypertriglyceridemia in human APOC1 transgenic mice is caused by apoC-I-induced inhibition of LPL.” J. Lipid. Res. 46 (2005) 297-306.
Birjmohun, R. S., G. M. Dallinga-Thie, J. A. Kuivenhoven, E. S.g. Stroes, J. D. Otvos, N. J. Wareham, R. Luben, J. J.p. Kastelein, K.-T. Khaw, and S. M. Boekholdt. "Apolipoprotein A-II Is Inversely Associated With Risk of Future Coronary Artery Disease." Circulation 116 (2007): 2029-035.
Boström et al. “A PGC1-α-Dependent myokine that drives brown-Fat-like development of white fat and thermogenesis.“ Nature., U.S. National Library of Medicine, 11 Jan. 2012
Brewer, H. B., S. E. Lux, R. Ronan, and K. M. John. "Amino Acid Sequence of Human ApoLp-Gln-II (apoA-II), an Apolipoprotein Isolated from the High-Density Lipoprotein Complex." Proceedings of the National Academy of Sciences 69.5 (1972): 1304-308.
Brewer, H. B., R. Shulman, P. Herbert, R. Ronan, and K. Wehrly. “The complete amino acid sequence of alanine apolipoprotein (apoC-3), and apolipoprotein from human plasma very low density lipoproteins.” J Biol Chem 249.15 (1974):4975-84.
Brewer, H. B., T. Fairwell, A. LaRue, R. Ronan, A. Houser, and T. J. Bronzert. “The amino acid sequence of human Apoa-I, an apolipoprotein isolated from high density lipoproteins.” Biochemical and Biophysical Research Communications 80.3 (1978):623-30.
Chen, S., G. Habib, C. Yang, Z. Gu, B. Lee, S. Weng, S. Cai, J. Deslypere, M. Rosseneu, and Al. Et. "Apolipoprotein B-48 Is the Product of a Messenger RNA with an Organ-specific In-frame Stop Codon." Science 238 (1987): 363-66.
Chen, S.H.; C. Y. Yang, P. F. Chen, d. Setzer, M, Tanimura, W. H. Li, A. M. Gotto, and L. Chan. “The Complete cDNA and Amino Acid Sequence of Human Apolipoprotein B-100.” J. Biol. Chem. 261 (1986): 12918-21.
Conde-Knape K., A. Bensadoun, J.H. Sobel. J.S. Cohn, and N.S. Shachter. “Overexpression of apoC-I in apoE-null mice: severe hypertriglyceridemia due to inhibition of hepatic lipase” J. Lipid Res. 43 (2002): 2136-2145.
Gaubatz, J.W., C. Heideman, A. M. Jr. Gotto, J. D. Morrisett, and G. H. Dahlen. “Human plasma lipoprotein [a]. Structural properties.” J. Biol. Chem. 258.7 (1983):4582-9.
Gaubatz, J.W., K. I. Ghanem, J. Jr. Guevara, M. L. Nava, W. Patsch, and J. D. Morrisett. “Polymorphic forms of human apolipoprotein[a]: inheritance and relationship of their molecular weights to plasma levels of lipoprotein[a].” J. Lipid Res. 31.4 (1990):603-13.
Guevara, J., J. Spurlino, A.y. Jan, C.y. Yang, A. Tulinsky, B.v. Prasad, J.w. Gaubatz, and J.d. Morrisett. "Proposed Mechanisms for Binding of Apo[a] Kringle Type 9 to Apo B-100 in Human Lipoprotein[a]." Biophysical Journal 64 (1993): 686-700.
Jackson, R.L., J. T. Sparrow, H. N. Baker, J.D. Morrisett, O. D. Taunton, and A. M. Jr. Gotto. “The primary structure of apolipoprotein-serine.” J. Biol. Chem. 249.16 (1974): 5308-13.
Jackson, R. L., h. N. Baker, E. B. Gilliam, and A. M. Jr. Gotto. “Primary structure of very low density apolipoprotein C-II of human plasma.” Proc Natl. Acad. Sci. U S A 74.5 (1977): 1942-5.
Jenny, N. S., N. D. Yanez, B. M. Psaty, L. H. Kuller, C. H. Hirsch, and R. P. Tracy. "Inflammation Biomarkers and Near-Term Death in Older Men." American Journal of Epidemiology 165 (2007): 684-95.
Kabagambe, E. K., S. E. Judd, V. J. Howard, N. A. Zakai, N. S. Jenny, M. Hsieh, D. G. Warnock, and M. Cushman. "Inflammation Biomarkers and Risk of All-Cause Mortality in the Reasons for Geographic and Racial Differences in Stroke Cohort." American Journal of Epidemiology 174 (2011): 284-92.
Koenig, W., M. Sund, M. Frohlich, H.-G. Fischer, H. Lowel, A. Doring, W. L. Hutchinson, and M. B. Pepys. "C-Reactive Protein, a Sensitive Marker of Inflammation, Predicts Future Risk of Coronary Heart Disease in Initially Healthy Middle-Aged Men: Results From the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 198." Circulation 99 (1999): 237-42.
Liu, M. and P.V. Subbaiah. “Activation of plasma lysolecithin acyltransferase reaction by apolipoproteins A-I, C-I and E.” Biochim. Biophys. Acta. 1168 (1993): 144-152.
Marsik, C., L. Kazemi-Shirazi, T. Schickbauer, S. Winkler, C. Joukhadar, O. F. Wagner, and G. Endler. "C-Reactive Protein and All-Cause Mortality in a Large Hospital-Based Cohort." Clinical Chemistry 54 (2008): 343-49.
Kones, Richard. "Rosuvastatin, Inflammation, C-reactive Protein, JUPITER, and Primary Prevention of Cardiovascular Disease – a Perspective." DDDT Drug Design, Development and Therapy 4 (2010): 383.
Poensgen, J., “Apolipoprotein C-1 inhibits the hydrolysis by phospholipase A2 of phospholipids in liposomes and cell membranes” Biochim. Biophys. Acta. 1042 (1990): 188-192.
Sehayek , E. , and S. Eisenberg . “Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway.” J. Biol. Chem. 266 (1991) : 18259 – 18267 .
Rall, S C, K H Weisgraber, T L Innerarity, T P Bersot, R W Mahley, and C B Blum. "Identification of a New Structural Variant of Human Apolipoprotein E, E2(Lys146 Leads to Gln), in a Type III Hyperlipoproteinemic Subject with the E3/2 Phenotype." Journal of Clinical Investigation 72.4 (1983): 1288-297.
Robbins, K. C., L. Summaria, B. Hsieh, and R. J. Shah. “The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin.” J Biol Chem. 242.10 (1967):2333-42.
Warden, C., C. Hedrick, J. Qiao, L. Castellani, and A. Lusis. "Atherosclerosis in Transgenic Mice Overexpressing Apolipoprotein A-II." Science 261 (1993): 469-72.
Wei, C. F., S. H. Chen, C. Y. Yang, Y. L. Marcel, R. W. Milne, W. H. Li, J. T. Sparrow, A. M. Gotto, and L. Chan. "Molecular Cloning and Expression of Partial CDNAs and Deduced Amino Acid Sequence of a Carboxyl-terminal Fragment of Human Apolipoprotein B-100." Proceedings of the National Academy of Sciences 82 (1985): 7265-269.
Yang, Chao-Yuh, and Pownall, H.J. "In Structure and Function of Plasma Apolipoproteins." Structure and Function of Apolipoproteins. Boca Raton, Fla.: CRC, 1992. Print.
Yang, Chao-Yuh, San-Hwan Chen, Sandra H. Gianturco, William A. Bradley, James T. Sparrow, Masako Tanimura, Wen-Hsiung Li, Doris A. Sparrow, Hans Deloof, Maryvonne Rosseneu, Fu-Shin Lee, Zi-Wei Gu, Antonio M. Gotto, and Lawrence Chan. "Sequence, Structure, Receptor-binding Domains and Internal Repeats of Human Apolipoprotein B-100." Nature 323 (1986a): 738-42.
Yang, Chao-Yuh, T. W. Kim, S. A. Weng, B. R. Lee, M. L. Yang, and A. M. Gotto. "Isolation and Characterization of Sulfhydryl and Disulfide Peptides of Human Apolipoprotein B-100." Proceedings of the National Academy of Sciences 87 (1990): 5523-527.
Yang, Chao-Yuh, Z. W. Gu, S. A. Weng, T. W. Kim, S. H. Chen, H. J. Pownall, P. M. Sharp, S. W. Liu, W. H. Li, and A. M. Gotto. "Structure of Apolipoprotein B-100 of Human Low Density Lipoproteins." Arteriosclerosis, Thrombosis, and Vascular Biology 9 (1989a): 96-108.
Yang, Chao-Yuh, Zi-Wei Gu, Lawrence Chan, Henry J. Pownall, and Antonio M. Gotto. "Structure and Functional Domains of Human Apolipoprotein B-100: A Strategy to Elucidate the Structure Information of a Large Protein." Methods in Protein Sequence Analysis (1989b): 466-74.
Zheng , C. , C. Khoo , K. Ikewaki , and F. M. Sacks . “ Rapid turnover of apolipoprotein C-III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions.” J. Lipid Res. 48 (2007): 1190 -203.