Epitope tags provide a convenient way to isolate interacting proteins without the need for specific antibodies to each new protein. The FLAG can be fused to N- or C-terminus of the protein and has been used in expression systems for the detection, quantification and purification of heterologous proteins in many biological systems including E.coli and mammalian systems. Anti-Flag tag antibody is a useful tool for the analysis of Flag tagged protein with different methods such as western blot, immunoprecipitation and flow cytometry.
ABSbio™ DYKDDDDK (flag) tag ELISA Kit provide a convenient, ready-to-use, high-throughput platform for rapid capture and detection of recombinant flag fusion proteins in different samples. The High Sensitivity plate is coated with the mouse monoclonal antibody (Binds to the same epitope as Sigma 'Anti-FLAG' M2 Antibody, Anti-FLAG is a trademark of Sigma-Aldrich) and pre-blocked to provide timesaving for high-throughput users. The monoclonal antibody is covalently bound to the plate in a favorable orientation such that the Fab region of the antibody is available for the DYKDDDDK (flag) tag to provide greater specificity. The anti-DYKDDDDK high sensitivity antibody coated multiwell plate can be used to detect N-terminal, Met-N-terminal, internal, and C-terminal flag and 3xflag fusion proteins. The kit can detect as little as 1 ng/well with a capacity of up to 300 ng/well of flag fusion protein.
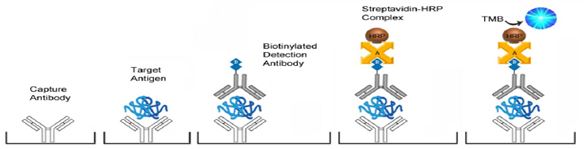
The kit uses a double‐antibody sandwich enzyme‐linked immunosorbent assay (ELISA) to analyze the level of flag-fusion protein in samples. First add standard and sample to wells pre‐coated with anti-DYKDDDDK (flag) antibody to capture available flag-fusion protein in solution, and perform incubating and washing procedures to remove unbound substance. Then add second Biotin‐conjugated anti-DYKDDDDK (flag) antibody to bind the captured protein, followed by another round of incubation and washing procedures to remove unbound antibody‐Biotin. Finally, streptavidin-HRP and TMB substrates are added, incubated for detection, and a blue color is developed. Reaction is stopped and color turns to yellow when Stopping Solution (acidic) is added. The yellow color intensity proportionally correlates to the concentration of the flag-fusion protein in samples.
试剂盒组分

储存及处理: Store standard & Detection Ab at -20°C, other components at 2-8°C. Shelf life: 3 months after receipt. Warm Reagents to room temperature before use.
应用:Measurement of flag fusion protein in serum, plasma, culture media and cell lysate.
反应种属:Human, Mouse, Rat
样品类型:serum, plasma, cell and tissue culture supernatant, and homogenate cell and tissue samples
检测方法:Colorimetric sandwich ELISA assay (OD450 nm)
检测时间:3-4 hrs
储存温度:-20°C & 2-8°C
保质期:3 months after receipt.
运输:Icepacks
所需其他材料:
Multi-channel pipette for washing
Cell culture incubator
Orbital shaker
micro plate reader
试剂准备
1. Wash Solution: 10x dilute Wash Solution with dH2O to prepare 1x Wash Solution.
2. Flag-fusion Protein Standard (3 vials): Each vial contains 12 μl of the standard sufficient for a 96-well plate. The undiluted standard can be stored at -20° C for up to 3 months if not used immediately. Spin to bring down the material prior to open the tube. Add 10 μl of the standard to 500 μl of Assay Diluent to make the high standard concentration of 2000 ng /ml. Vortex briefly and allow it to sit for a minimum of 15 min prior to use. A seven point standard curve is generated using 2-fold serial dilutions in Assay Diluent, vortex briefly for each of dilution step. Store the rest of the standard at -20° C.
3. Detection Ab: Immediately before use, add 50 μl of the antibody into 11 ml of Assay Diluent for one plate (for partial plate assay, adjust the volumes accordingly).
4. Streptavidin-HRP: Immediately before use, add 50 μl of the Streptavidin-HRP into 11 ml of Assay Diluent for one plate (for partial plate assay, adjust the volumes accordingly).
检测程序
1. It is recommended that all standards and samples should be run in duplicate. Prepare sample dilutions (10 fold series dilition) in a clean 96‐well plate with 1x PBS. Set standard wells, testing sample and blank wells on the assay plate/strip. Transfer diluted standard 100 μl to standard wells, diluted sample 100 μl to sample wells, assay diluent 100 μl only to blank wells.
2. Cover the plate with plate sealer and incubate the plate at room temperature for 2 hrs or at 37 °C for 1h, shaking the plate at 600-700 rpm on a micro-plate shaker.
3. Decant as much liquid as possible, fill the wells with 200 μl wash solution, shaking for 1 min, decant the wash solution and remove residual liquid with absorbent paper. Repeat wash three times.
4. Add 100 µl of 1x Detection antibody solution per well. Cover the plate with plate sealer and incubate the plate at room temperature for 1h, shaking the plate at 600-700 rpm on a micro-plate shaker.
5. Wash 3 times as outlined in step 3.
6. Add 100 µl of 1xStreptavidin-HRP solution per well. Cover the plate with plate sealer and incubate the plate at room temperature for 30 minutes, shaking the plate at 600-700 rpm on a micro-plate shaker.
7. Wash 5 times as outlined in step 3.
8. Add 100 µl of TMB Solution to each well and incubate at room temperature for 10~20 minutes protect from light, or keep close monitoring on the developing process until desired developing blue color observed. Note: please be aware that the color may develop more quickly or more slowly that the recommended incubation time depending on the localized room temperature.
9. Add 100 µl of Stop Solution to each well to stop the reaction (the blue color change to yellow), gently tap the plate frame for a few seconds to ensure thorough mixing.
10. Read absorbance of the plate on a microplate reader at 450 nm within 15 min.
11. Average the duplicate readings for each standard and samples, subtract the average zero (blank) standard optical density. Construct standard curve (plotting the mean OD450 for each standard on the X‐axis against concentration on the Y‐axis, draw a best‐fit curve through the points) and calculate linear regression equation, then use corrected sample OD values and regression equation to calculate the corresponding sample concentration. It should be remembered that the sample has been diluted and its actual concentration should be justified by dilution factor (the measurement and calculation can be accomplished by software like SoftMax).
12. If molecular weight of sample differs from flag fusion protein standard (MW 49.4kD) apply the following equation to the reading concentration to obtain the actual concentration = [MW Sample]/[MW protein standard] x Sample reading (ng/mL)
分析特点
灵敏度:The lowest FLAG fusion protein level that can be measured by this assay is 1 ng/well (20ng/mL).
精确度:Intra-assay Precision (Precision within an assay) C.V. < 10%. Inter-assay Precision (Precision between assays) C.V. <10%.
回收率:The recovery of the assay was determined by adding various amounts flagG fusion protein to a sample. The measured concentration of the spiked sample in the assay was compared to the expected concentration. The average recovery was 96-103 %.
特异性:Cross reactivity with N-terminal, Met-N-terminal, internal, and C-terminal FLAG and 3xFLAG fusion proteins.